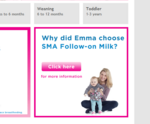
The complaints relate to three national press adverts and three posters for the product, which is manufactured by Pfizer. Each advert featured a mother and child and a headline that incorporated the name of the mother in the ad.
“Kate breastfed her son because she knew it would give him the best start in life. When she finished, she was determined to find the best option for her baby,” said one of the ads, adding: “For Kate, the best milk after hers is SMA Follow-on Milk.”
Objections were received from 64 complainants including UNICEF UK, the Royal College of Paediatrics and Child Health, Baby Milk Action and the Lactation Consultants of Great Britain.
The complainants challenged the ads on four points – of which two were upheld by the ASA.
Misleadingly implied
The petitioners challenged whether the posters misleadingly implied that follow-on milk was the best alternative to breast milk and superior to other follow-on milk products.
These complaints were upheld by the ASA.
“Because we did not see evidence that SMA Follow-on Milk was the best alternative to breast milk once breastfeeding had stopped, or that it was superior to other follow-on milk, we concluded that the ads were misleading on these grounds,” said the ASA decision.
The complainants also challenged whether the ads misleadingly implied that breastfeeding should stop at six months, and questioned if the ads sufficiently differentiated between infant formula and follow-on formula.
The ASA concluded that these points were not misleading, but ordered Pfizer to make necessary adjustment to the ads, stating: “The ads must not appear again in their current form.”
Pfizer disappointed
Pfizer has voiced its disappointment in the ruling, stating that it takes compliance with advertising laws very seriously.
“SMA Nutrition takes compliance very seriously and as such is disappointed that two of the four complaints made against the SMA Follow-on Milk advertising have been upheld. The adverts were designed to convey the personal opinion of real Mums and why SMA Follow-on Milk was the best choice for those particular individuals after breastfeeding had stopped.”
“The ASA did not feel that the advertising portrayed personal testimonials and that the average consumer would not recognise this. SMA Nutrition can confirm that the advertising is no longer in use.”
One of the complainants, Baby Milk Action has welcomed the decision, but fears “the damage has already been done.”
“The ASA ruling shows that SMA formula is not the best milk after Kate’s. This ruling comes after the damage has already been done and without fines won’t deter future malpractice, but it does prove the point that Pfizer/Wyeth’s national advertising campaign misled parents,” said Baby Milk Action campaigns and networking coordinator, Mike Brady.







