Voluntary Withdrawal of Antimicrobials for Use in Food-Producing Animals


Published: 2014-04-15 Views:
10
Core Tip: The US Food and Drug Administration is today announcing that five drug sponsors holding animal drug applications affected by Guidance For Industry (GFI) #213 have requested that FDA withdraw approval of a collective 19 animal drug applications because the
The US Food and Drug Administration is today announcing that five drug sponsors holding animal drug applications affected by Guidance For Industry (GFI) #213 have requested that FDA withdraw approval of a collective 19 animal drug applications because the products are no longer manufactured or marketed.
Of these 19 applications, 16 are antimicrobials affected by GFI #213. The guidance outlines FDA’s plan to help curb antimicrobial resistance by, among other things, phasing out the use of medically important antimicrobials in food-producing animals for production purposes.
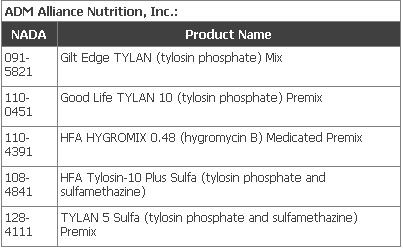
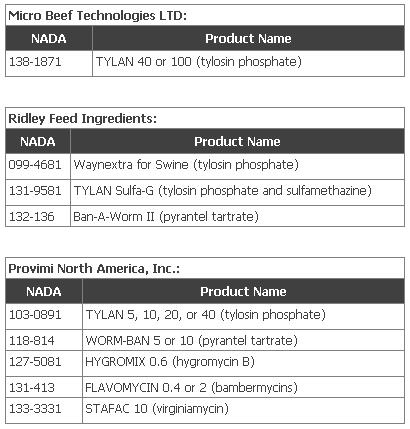
The following companies have requested that FDA withdraw approval for their listed products:
[ News search ]
[ ]
[ Notify friends ]
[ Print ]
[ Close ]