The outbreak stemmed from a bad batch of romaine lettuce grown in Arizona’s Yuma region, according to an investigation by the Centers for Disease Control. The lettuce was contaminated with E. coli O157:H7, a pathogenic strain of bacteria that officials also found in the region’s canal water.
Government agencies are still searching for the source of the bacteria as contaminants have many ways of slipping past detection.
Government agencies are still searching for the source of the bacteria as contaminants have many ways of slipping past detection.
Laws require that farmers regularly send irrigation water samples to certified labs for safety testing. These labs use sensitive tests but they take about a day or two to run.
Now, a team of researchers at Cornell University, Iowa State University, and the organization Global Good/Intellectual Ventures has developed a sensitive, bacteriophage-based technique to detect one particular E. colistrain in water in less than half a day (Analyst 2018, DOI: 10.1039/c8an00781k). Study coauthor Sam Nugen of Cornell presented the work during a talk in the Division of Agricultural and Food Chemistry at the American Chemical Society national meeting in Boston on Wednesday.
Found in great numbers all over our bodies, bacteriophage viruses are harmless to humans yet lethal to bacteria, Nugen said. Bacteriophages prey on bacteria by hijacking the bacteria’s internal machinery to express the phage’s own DNA and then forcing them to self-destruct.
To adapt phages’ bacteria-killing abilities for bacteria detection, the researchers inserted the gene for a reporter enzyme into a bacteriophage called T7NLC. The reporter enzyme, a luciferase known as NanoLuc, glows when expressed by bacterial cells, thus detecting E. coli. Fused to the enzyme is a module that binds to cellulose to provide a way to collect the enzymes for analysis.
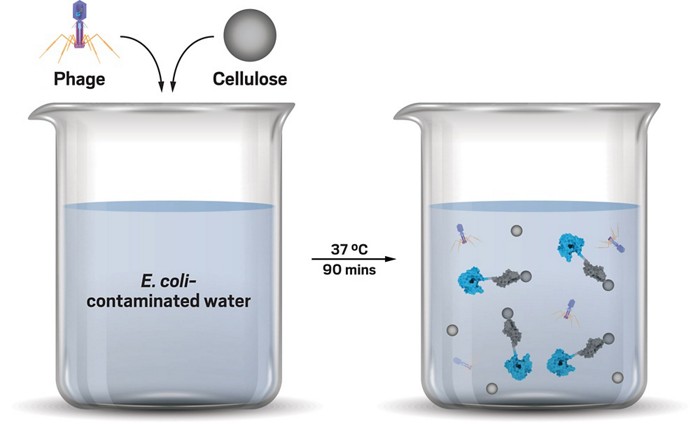
When researchers added the engineered bacteriophages to E. coli contaminated water along with insoluble cellulose pellets, the phages infected the bacterial cells. After the bacteria expressed the luminescent reporter enzyme, the cells disintegrated, freeing the enzyme, which clung to the cellulose pellets. Decanting off the water quickly concentrated samples. Using a laboratory plate counter or normal camera set to 30-second exposure time, scientists quantitatively measured the presence of under 10 colony forming units of E. coli per 100 mL within three hours.
It’s an elegant design to overcome some of the limitations others have encountered when trying to sensitively detect cells using bioluminescent reporter phage, said Cath Rees, a microbiologist at University of Nottingham. Previous approaches have required extended enrichment times, which slows down testing. However, the newly engineered phage needs to be able to detect a much broader range of bacteria to be applicable in a real-world setting, she said. The majority of E. coli strains are not pathogenic but they can still be good indicators that water is contaminated.
Nugen said the team is tailoring the phage to detect pathogenic E. coli as well as bacteria that cause salmonella. The scientists have also developed a quantitative version of the technology that uses a cellulose filter that traps both the bacteria and phages. This version is able to detect a single colony forming unit of bacteria but takes about 10 hours. Nugen presented this work during a session in the Division of Environmental Chemistry on Sunday.