The amount of mercury in "Zhuifeng Tougu Wan" may present a significant health risk to Canadians, including vulnerable populations such as children and pregnant women who are most susceptible to the toxic effects. Symptoms include nausea, abdominal pain, vomiting, muscle cramps, diarrhea, heart abnormalities, anaemia, liver, and nervous system problems.
Who is affected:
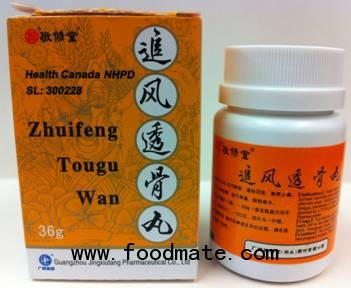
Canadians who have purchased "Zhuifeng Tougu Wan".
What consumers should do:
Speak to your healthcare practitioner about any questions or concerns regarding this product.
Contact the company, Wing Quon Enterprises, at 1-604-273-5028 for information on the recall of this product.
Report any adverse reaction you suspect may be related to this product to Health Canada (see below).
Read the label of the products you buy to verify that they have been assessed by Health Canada for safety, effectiveness and quality.
What Health Canada is doing:
Health Canada is monitoring the voluntary recall by Wing Quon Enterprises, who distributed "Zhuifeng Tougu Wan", indicated for traditional Chinese use, to four retail locations in B.C (Guang Zhou Herbs Plus in Chilliwack, De Xing Long in Vancouver, Nam Pek Enterprises Ltd. in Vancouver and Richmond, B.C) and Sun Ming Hong in Toronto, ON.
Background:
Health products that have been authorized for sale by Health Canada will have an eight-digit Drug Identification Number (DIN), a Homeopathic Medicine Number (DIN-HM) or a Natural Product Number (NPN) on the label. Some natural health products may have an Exemption Number (EN), which indicates that the product is legally available for sale while Health Canada is reviewing its application for licensing.
Health Canada's acceptable limit for exposure to mercury and lead in a natural health product is the same for both substances: 0.29 micrograms per kilogram of body weight per day. Health Canada's acceptable limit for exposure to arsenic in a natural health product is 0.14 micrograms per kilogram of body weight per day.
The toxic effects of lead may cause abdominal pain, anemia, changes in blood pressure, reproductive effects, weakness, concentration problems, weight loss, insomnia, dizziness, kidney and brain damage, and ultimately death. The toxic effects of arsenic include skin and lung cancers, heart and lung diseases, and brain damage.
Affected Product:
Zhuifeng Tougu Wan

For more information
Consumers and health professionals wanting more information about this advisory from Health Canada can contact the Public Enquiries Line at 613-957-2991, or toll free at 1-866-225-0709.
Media enquiries related to this Advisory should be directed to Health Canada Media Relations at 613-957-2983.
How to report problems with consumer products:
Call toll-free at 1-866-234-2345
Visit MedEffect Canada's web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax





