PHOs, or partially hydrogenated oils, have been used as ingredients since the 1950s to improve the shelf-life of processed foods. FDA has issued a final determination that PHOs, the primary source of industrially produced trans fat in processed foods, are not “Generally Recognized as Safe,” or GRAS. This means that PHOs may no longer be added to food after June 18, 2018, unless they are otherwise approved by FDA.
In this case, it has become clear that what’s good for extending shelf-life is not equally good for extending human life. A 2002 report by the National Academy of Sciences’ Institute of Medicine found a direct correlation between intake of trans fat and increased levels of low-density lipoprotein (LDL) cholesterol. LDL cholesterol is commonly known as “bad” cholesterol because it contributes to clogged, damaged arteries.
What this means is that there is an increased risk of heart disease, so much so that this action is expected to reduce coronary heart disease and prevent thousands of fatal heart attacks each year.
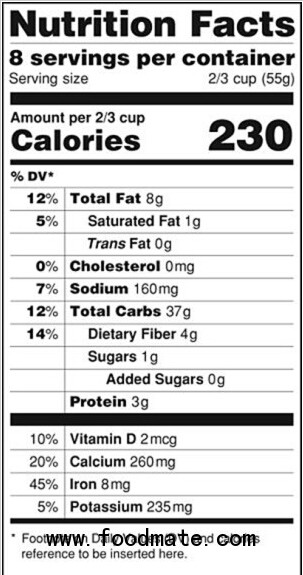
In 2006, FDA required that manufacturers declare the amount of trans fat on the Nutrition Facts label because of these public health concerns. Many manufacturers responded by voluntarily changing their product formulations to reduce or eliminate trans fat, and consumers started avoiding foods with trans fat.
Despite the declines in trans fat in foods, PHOs continue to be found in some brands of popular food products, such as frostings, microwave popcorn, packaged pies, frozen pizzas, stick margarines and coffee creamers. And for consumers who consistently choose products with added PHOs, their daily intake of industrially produced trans fat is approximately twice as high as the average consumer. Today, FDA has issued its determination that PHOs are not generally recognized as safe.
We are establishing a three-year compliance period. This will allow for an orderly process as companies make the transition to reformulate products and, if they choose, to allow companies or other interested parties to use the food additive petition process to present evidence to FDA as to whether any uses of PHOs meet our standard for safety. Thus, industry is responsible for providing evidence to FDA to demonstrate safety, while FDA is responsible for evaluating that evidence to determine whether to approve PHOs for any specific intended use.
We know that many companies have already removed PHOs, and we expect that others will accelerate the phasing out of PHOs based on today’s action. FDA encourages consumers seeking to reduce trans fat intake to check the Nutrition Facts label for trans fat. The most effective way to avoid PHOs is to check the ingredient list for partially hydrogenated oils. Even if trans fat is listed as “0”, some PHOs could be in the product.
At the heart of FDA’s mission is a responsibility to ensure that the foods we eat and share with our family are as safe as possible. It’s a responsibility to protect health by taking action when needed, based on the best available science. This action will ultimately allow all of us to enjoy safer foods and healthier lives.